OraSure OraQuick®
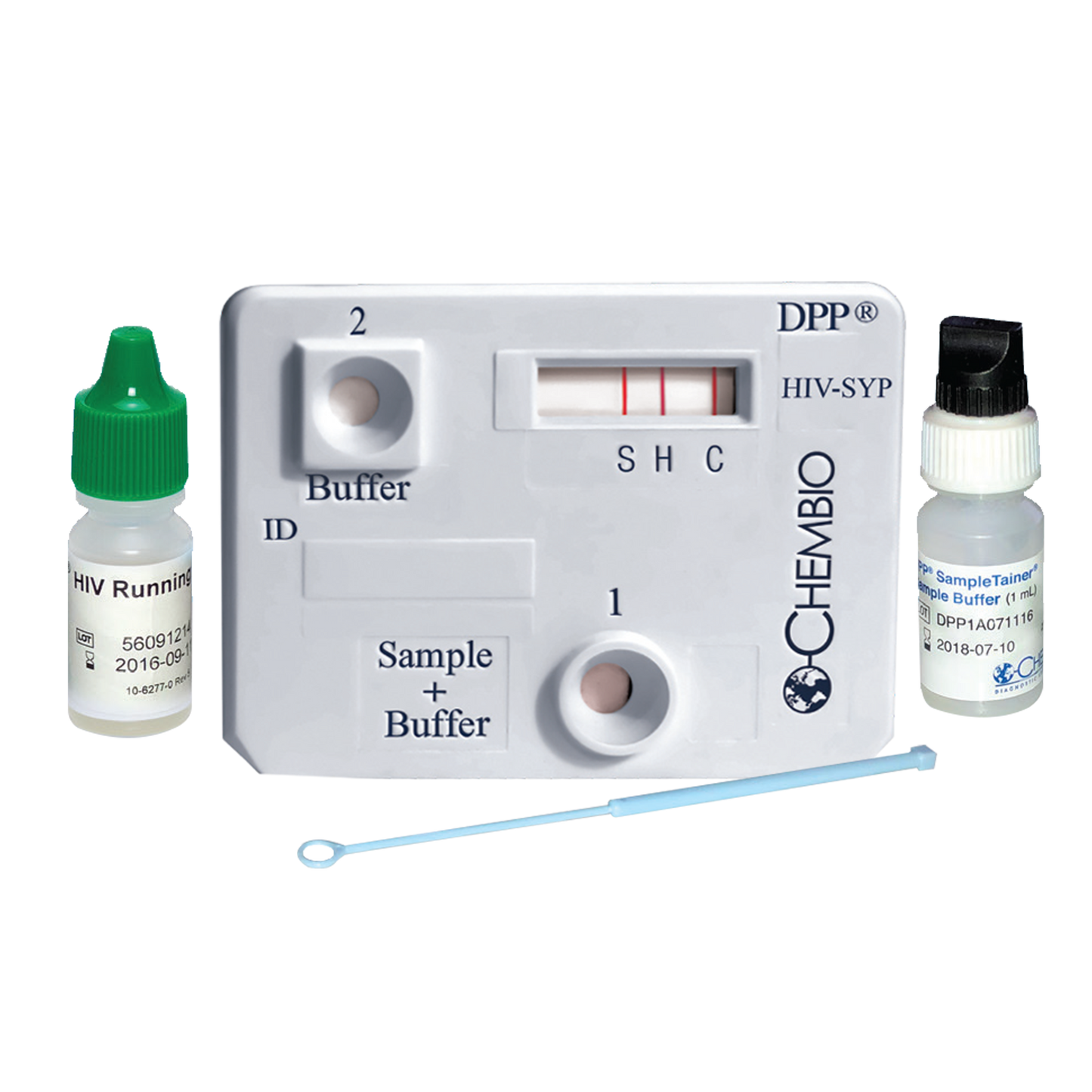
CHEMBIO DPP® HIV-Syphilis Test Kit – 20 Tests/Kit
CHEMBIO DPP® HIV-Syphilis Test Kit – 20 Tests/Kit
Couldn't load pickup availability
CHEMBIO DPP® HIV-Syphilis Test Kit – 20 Tests/Kit
The CHEMBIO DPP® HIV-Syphilis Test is a rapid, multiplex, point-of-care immunoassay designed for the simultaneous detection of antibodies to HIV-1/2 and Treponema pallidum (syphilis). This dual-path platform (DPP®) uses a small blood sample and provides fast, accurate results in just 15–20 minutes—making it ideal for clinics, outreach programs, and public health screenings.
Key Features:
- Dual Detection: Simultaneously screens for HIV-1/2 and syphilis antibodies in a single test
- Rapid Results: Results available in 15–20 minutes
- Sample Types: Uses fingerstick whole blood, venous whole blood, or plasma
- User-Friendly: Simple three-step process with minimal training required
- Portable & Flexible: Compact format and no refrigeration required; test can be conducted in various healthcare or community settings
- Objective Results: Compatible with the DPP® Micro Reader for enhanced result accuracy and digital recordkeeping
- CLIA-waived & FDA-cleared for use in low-complexity settings
Performance:
- HIV Sensitivity: >99%
- HIV Specificity: >99%
- Syphilis Sensitivity: ~94–95%
- Syphilis Specificity: ~95–96%
Kit Contents (20 Tests):
- 20 individually sealed test devices
- 20 specimen collection loops (10 µL)
- 1 vial of running buffer
- 20 buffer tubes with caps (SampleTainer® format)
- 1 set of detailed instructions for use (IFU)
Note: DPP® Micro Reader and external controls are available separately.
Storage & Shelf Life:
- Storage Temperature: 2°C to 30°C (36°F to 86°F)
- Shelf Life: Up to 24 months from manufacture date
- Test Operating Temperature: Room temperature (18–30°C)
Ideal For:
- STD and HIV clinics
- College and university health programs
- Urgent care centers
- Community health screenings
- Global health initiatives
The CHEMBIO DPP® HIV-Syphilis test kit is a cost-effective, time-saving solution that helps healthcare professionals provide comprehensive screening in one easy step—supporting early detection, prevention, and care.
Share